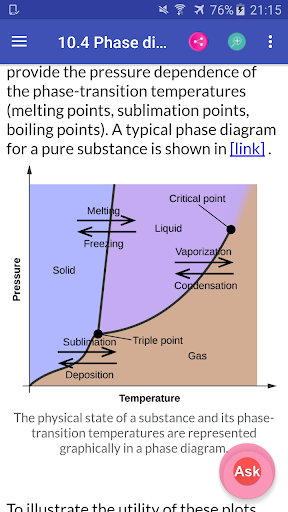
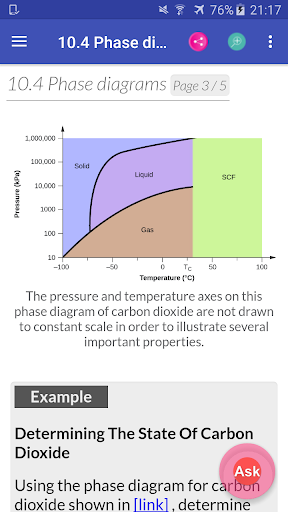
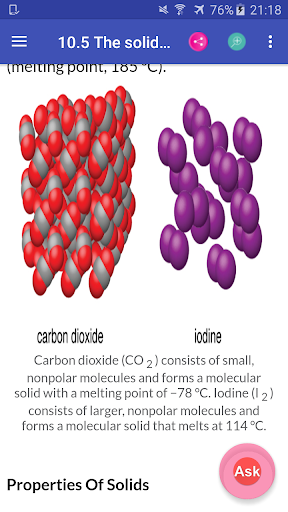
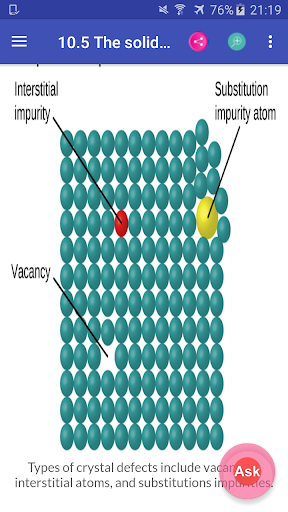
Chemistry Interactive Textbook app
free Chemistry Interactive Textbook app
download Chemistry Interactive Textbook app
Chemistry Interactive Textbook apk
free Chemistry Interactive Textbook apk
download Chemistry Interactive Textbook apk

Chemistry Interactive Textbook
4.2
50K+
ADVERTISEMENT
Screenshots
Comment
Similar Apps
Top Downloads
Copy [email protected]. All Rights Reserved
Google Play™ is a Trademark of Google Inc.
Apkguides is not affiliated with Google, Android OEMs or Android application developers in any way.